Section 1: Calculating the Molality
If you know the number of moles of a component present and the mass of solvent that they are in, you can find the molality of the solution.
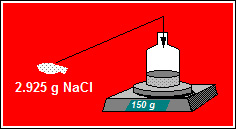
150 grams of water contains 2.925 grams of Sodium Chloride. What is the molality of Sodium Chloride (RMM 58.5) in the solution? Answer to 4 significant figures.
Click to Remove Question
The molality of Sodium Chloride in the solution is 0.3333 molal.
Molality = Number of Moles of Component
Mass of SOLVENT (in kgs)
Mass of SOLVENT (in kgs)
- Find the moles of Sodium Chloride present:
Moles = Mass / RMM = 2.925 / 58.5 = 0.05 moles of NaCl - Convert the mass of water from grams to kilograms:
150 g = 0.15 kg - Find the molality of Sodium Chloride
Molality of NaCl = 0.05 moles/kg
0.15
Molality of NaCl = 0.33333 = 0.3333 moles/kg to 4 sig figs