Section 3: Calculating the Mass of Solvent
If you know the molality and the number of moles of a component present, you can calculate the mass of solvent involved.
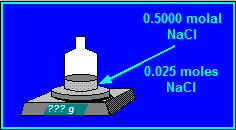
What is the mass of water (in grams) associated with 0.025 moles of Sodium Chloride if the molality of Sodium Chloride in the solution is 0.5000 molal?
Click to Remove Question
50 grams of water are associated with 0.025 moles of Sodium Chloride.
Molality = Number of Moles of Component
Mass of SOLVENT (in kgs)
Mass of SOLVENT (in kgs)
which rearranges to:
Mass of Solvent (in kgs) = Number of Moles of Component
Molality of Component
Mass of water = 0.025 gMolality of Component
0.5000
Mass of water = 0.05 kg
Mass of water = 50 g
Click to Continue