Section 2: Calculating the Number of Moles
If you know the molality of a component and the mass of solvent involved, you can calculate the number of moles of the component.
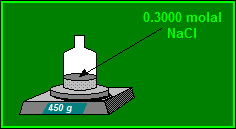
A pharmacist prepares a 0.3000 molal solution of Sodium Chloride. If the pharmacist uses 450 grams of water, how many moles of Sodium Chloride are present? Answer to 3 decimal places.
Click to Remove Question
0.135 moles of Sodium Chloride are present.
which rearranges to:
Number of Moles of Component = Molality x Mass of Solvent (in kgs)
Convert mass of water from grams to kilograms: 450 g = 0.45 kg
Moles of Sodium Chloride = 0.3000 x 0.45
Moles of Sodium Chloride = 0.1350 moles
Moles of Sodium Chloride = 0.135 moles to 3 dec pl
Molality = Number of Moles of Component
Mass of SOLVENT (in kgs)
Mass of SOLVENT (in kgs)
which rearranges to:
Number of Moles of Component = Molality x Mass of Solvent (in kgs)
Convert mass of water from grams to kilograms: 450 g = 0.45 kg
Moles of Sodium Chloride = 0.3000 x 0.45
Moles of Sodium Chloride = 0.1350 moles
Moles of Sodium Chloride = 0.135 moles to 3 dec pl
Click to Continue