Preparing a Solution of Known Molality
To make a solution of known molality, you add the required mass of solute to a small volume of the solvent and then make the final mass up to the total mass required.
The total mass is the mass of the solute PLUS the mass of solvent.
NOTE: you are adding two MASSES together.
To prepare a 0.3200 molal solution of Sucrose in water:
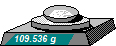 1 Calculate the mass of Sucrose needed
1 Calculate the mass of Sucrose needed
Mass = Moles x RMM = 0.32 x 342.3 g = 109.536 g
Weigh out 109.536 g of Sucrose.
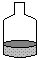 2 Dissolve the Sucrose in a little water.
2 Dissolve the Sucrose in a little water.
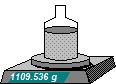 3 Add a total of 1000 grams of water ie make up the final mass to 1109.536 grams.
3 Add a total of 1000 grams of water ie make up the final mass to 1109.536 grams.
Mass = Moles x RMM = 0.32 x 342.3 g = 109.536 g
Weigh out 109.536 g of Sucrose.
 2 Dissolve the Sucrose in a little water.
2 Dissolve the Sucrose in a little water.
 3 Add a total of 1000 grams of water ie make up the final mass to 1109.536 grams.
3 Add a total of 1000 grams of water ie make up the final mass to 1109.536 grams.
Click to Continue
Further Information on Molality
- Masses do not change with temperature. Therefore if a solution's concentration is given as a molality, the concentration will stay the same at all temperatures. Compare this with molaRity in which concentration is expressed as moles per litre ie moles per a volume. As the temperature changes, the volume will change and therefore the molarity will also change: molarity varies with temperature.
- The molality of a component in a solution remains the same even if a second solute is added. This is because the mass of solvent involved remains the same. However, the molaRity of the component changes because the overall volume changes.
- Practically, it is easier to measure volumes - therefore solutions tend to be prepared in terms of moles per volume rather than moles per mass.
Click to Continue