Section 3: How much reactant do you need?
You will sometimes need to make a certain amount of a product and for this you will need to know how to calculate the mass of reactants required.
You can calculate the MINIMUM amount of reactant you need by assuming that a reaction occurs exactly as seen in the reaction equation. Look at the example below.
You can calculate the MINIMUM amount of reactant you need by assuming that a reaction occurs exactly as seen in the reaction equation. Look at the example below.
This is the MINIMUM amount to add because you are assuming that all the reactant is converted to product ie you have a percentage yield of 100 %. This very rarely happens.
The calculated figure tells you that you can not add LESS than that amount to obtain the required amount of product.
The calculated figure tells you that you can not add LESS than that amount to obtain the required amount of product.
You need to prepare 1 mole of Ethyl Ethanoate from the reaction:
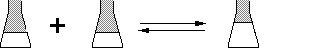
CH3COOH + C2H5OH ---------» «--------- CH3COOC2H5 + H20
1 mole1 mole1 mole
1 mole1 mole1 mole
From the balanced equation you can see that you need a minimum
of 1 mole of Ethanoic Acid and a minimum of 1 mole of Ethanol.
of 1 mole of Ethanoic Acid and a minimum of 1 mole of Ethanol.
Click to Continue
Click to Continue