For the next question, click on the Next Question button.
Question 1 of 3
What is the percentage yield of Ethyne in the following reaction:
-
Find the theoretical yield of Ethyne.
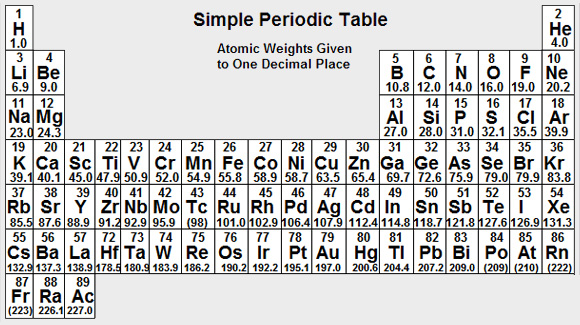
- Find the moles of Calcium Carbide used, where Moles = Mass/RMM RMM = 64.1 Moles of Calcium Carbide used = 10/64.1 = 0.15600 moles
- Find the theoretical volume of Ethyne that could be produced. Use the equation:
1 mole of Calcium Carbide could produce 1 mole of Ethyne. Thus:
0.15600 moles of Calcium Carbide could produce 0.15600 moles of Calcium Carbide
At 0°C and 760 mm Hg, 1 mole of Ethyne has a volume of 22.4 litres. Thus:
0.15600 moles of Ethyne has a volume of 0.15600 x 22.4 litres = 3.4945 litres
-
Find the percentage yield of Ethyne.
Percentage Yield = Actual Yield/Theoretical Yield x 100 %
Percentage Yield of Ethyne = 3 / 3.4945 x 100 % = 85.84 % = 85.8 % to 1 dec pl
No, that is not the correct answer.
Have another go: just type in your new answer and press Enter.
If you continue to have problems, have a look at the answer.
For the next question, click on the Next Question button.
Question 2 of 3
0.76 grams of Potassium Sulphate are produced using 70 ml of 0.2000 M Potassium Hydroxide and 46 ml of 0.1000 M Sulphuric Acid in the following reaction:
-
Find the theoretical yield of Potassium Sulphate.
- Find which of the reactants is limiting.
Molarity = Moles/Volume (in litres): Moles = Molarity x Volume (in litres)
Moles of Potassium Hydroxide = 0.2000 x 0.070 = 0.014 moles
Moles of Sulphuric Acid = 0.1000 x 0.046 = 0.0046 moles
From the balanced equation you can see that:
2 moles of Potassium Hydroxide could produce 1 mole of Potassium Sulphate. Thus:
0.014 moles of Potassium Hydroxide could produce 0.014/2 = 0.007 moles of Sulphate
1 mole of Sulphuric Acid could produce 1 mole of Potassium Sulphate. Thus:
0.0046 moles of Sulphuric Acid could produce 0.0046 moles of the Sulphate. - Theoretical Mass of Sulphuric Acid = Moles x RMM = 0.0046 x 174.2 g = 0.80132 g
- Find which of the reactants is limiting.
-
Find the percentage yield of Potassium Sulphate.
Percentage Yield = Actual Yield/Theoretical Yield x 100 = 0.76/0.80132 x 100 = 94.8 %
No, that is not the correct answer.
Have another go: just type in your new answer and press Enter.
If you continue to have problems, have a look at the answer.
Question 3 of 3
111 grams of Propanoic Acid reacts with 105 grams of Propanol to produce 113 grams of Propyl Propanoate. What is the percentage yield of Propyl Propanoate? Answer to 1 decimal place.
-
Find the theoretical yield of Propyl Propanoate.
- Find which of Propanoic Acid and Propanol is the limiting reactant.
Moles of Propanoic Acid = Mass/RMM = 111/74.0 = 1.5 moles RMM = 74.0
Moles of Propanol = Mass/RMM = 105/60.0 = 1.75 moles RMM = 60.0
From the balanced equation, you can see that:
1 mole of Propanoic Acid could produce 1 mole of Propyl Propanoate. Thus:
1.5 moles of Propanoic Acid could produce 1.5 moles of Propyl Propanoate.
1 mole of Propanol could produce 1 mole of Propyl Propanoate. Thus:
1.75 moles of Propanol could produce 1.75 moles of Propyl Propanoate. - Theoretical Mass of Propyl Propanoate = Moles x RMM = 1.5 x 116.0 = 174 g
- Find which of Propanoic Acid and Propanol is the limiting reactant.
-
Find the percentage yield of Propyl Propanoate.
Percentage Yield = Actual Yield/Theoretical Yield x 100 %
Percentage Yield = 113/174 x 100 % = 64.94 % = 64.9 % to 1 dec pl
No, that is not the correct answer.
Have another go: just type in your new answer and press Enter.
If you continue to have problems, have a look at the answer.