Section 1 contd: Preparing Solutions
In pharmacy, you often have to prepare weaker solutions from stronger solutions. You must be able to calculate how much of the stronger solution you need to prepare the weaker solution: you must know how to do dilutions.
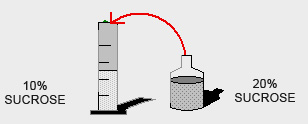
How many millilitres of a 20% w/v Sucrose solution do you need in order to prepare 500ml of a 10% w/v Sucrose solution?
Click to Continue
To solve these types of problems, you can use the same two approaches already met. This is because the quantity of the strong solution used will contain the same amount of the component that is present in the final weaker solution.
You will be shown how to solve one problem on the next screen.