The question that you will be shown how to solve is:
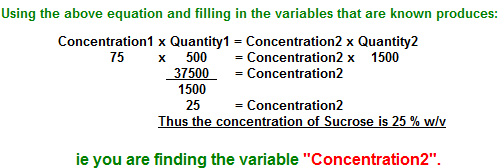
"If 500ml of a 75% w/v aqueous solution of source are diluted to 1500ml, what will be the new % w/v concentration of the Sucrose solution?"
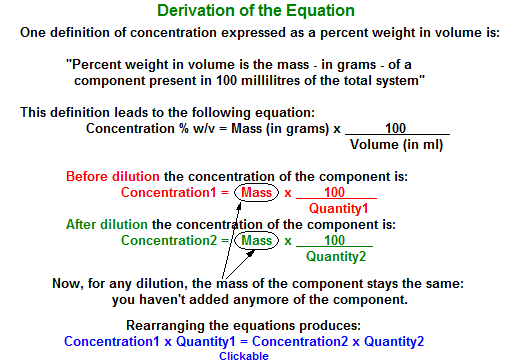
The long approach uses mainly the definition of percent weight in volume:
Percent weight in volume is the mass - in grams - of a component present in 100 millilitres of the total system".
"If 500ml of a75 % w/v aqueous solution of Sucrose are diluted to 1500ml, what will be the new % w/v concentration of the Sucrose solution?"
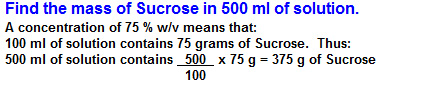


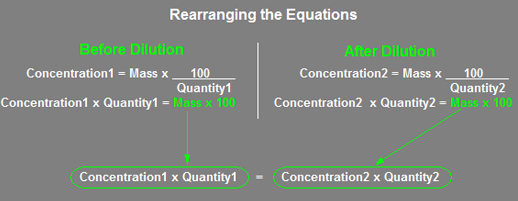
The short approach uses the following equation:
![]()
| Concentration1 | = Concentration of Original Solution |
| Quantity1 | = Quantity (mass or volume) of Original Solution |
| Concentration2 | = Concentration of New Solution |
| Quantity2 | = Quantity (mass of volume) of New Solution |
Click on Equation to Find Out Where It Comes From
"If 500ml of a 75% w/v aqueous solution of Sucrose are diluted to 1500ml, what will be the new % w/v concentration of the Sucrose solution?"