Section 1: Dilution and Concentration of Liquids
Liquids/solutions do not tend to come in the concentration that you want to use. You must therefore know how to calculate the concentration of liquids/solutions after they have been diluted or concentrated and you must also know HOW to dilute or concentrate solutions.
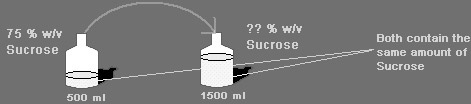
If 500 ml of a 75 % w/v aqueous solution of Sucrose are diluted to 1500 ml, what will be the new % w/v concentration of the Sucrose solution?
Click to Continue

On the next screen, you will be shown two approaches for solving such calculations. Both approaches use the fact that the amount of a component in the system IS THE SAME before and after dilution.