Section 2: Other Stock Solutions
You need to be able to calculate the volume needed of other stock solutions - such as Strong Potassium Chloride Solution and Benzalkonium Chloride. You can use the same methods as you used for the Ethanol calculations.
For example. How many millilitres of Benzalkonium Chloride - supplied as a 5 % w/v solution - do you need to prepare 450 ml of a 1 % solution?
For example. How many millilitres of Benzalkonium Chloride - supplied as a 5 % w/v solution - do you need to prepare 450 ml of a 1 % solution?
of a component in 100 millilitres of the total system.
100 ml of solution contains 1 g of Benzalkonium Chloride. Thus:
450 ml of solution contains 450/100 x 1 g = 4.5 g of Benzalkonium Chloride.
5 g of Benzalkonium Chloride in 100 ml of solution. Thus there are:
4.5 g of Benzalkonium Chloride in 4.5/5 x 100 ml = 90 ml of solution.
You need 90 ml of the 5 % Benzalkonium Chloride solution.
90.0 ml Benzalkonium Chloride Solution (5 %) to 450 ml Water
For example. How many millilitres of Benzalkonium Chloride - supplied as a 5 % w/v solution - do you need to prepare 450 ml of a 1 % solution?
Derivation of the Equation
One definition of concentration expressed as a percent weight in volume is:
"Percent weight in volume is the mass - in grams - of a
component present in 100 millilitres of the total system"
This definition leads to the following equation:
Volume (in ml)
you haven't added anymore of the component.
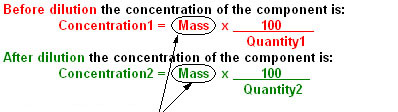
Rearranging the Equations
Before Dilution
Quantity1
After Dilution
Quantity2