You may find sometimes that a solution being made has a diluent added. This diluent WILL affect the concentration of the component under consideration and should be dealt with as if it had a zero concentration.
Diluents have a concentration of 0%.
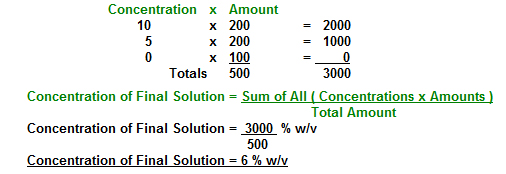
200 ml of 10 % (w/v) Ammonia and 200 ml of 5 % Ammonia are mixed together and the volume is made up to 500 ml with water. What is the concentration of the resulting solution?
Click here to remove Question
The water is a diluent and therefore has a concentration of 0 % Ammonia.
The volume of water that will be added is 500 - (200 + 200) ml ie 100 ml. The concentration of the final solution is found from the following:
Click here to continue