Where:
Parts of First Source =Required Concentration-Concentration of Second Source
and
Parts of Second Source= Concentration of First Source- Required Concentration
For the next question, click on the Next Question button.
Question 1 of 6
A solution is made from 200ml of 10% (w/v) Sodium Chloride solution, 350ml of 15% Sodium Chloride solution and 450ml of 25% Sodium Chloride solution. What is the concentration of the final solution? Answer to 1 decimal place.
Type in your answer and PRESS ENTER.
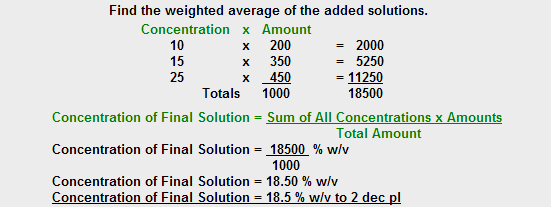
No, that is not the correct answer.
Have another go: just type in your new answer and press Return.
If you continue to have problems, click on the Answer button to see the answer.
For the next question, click on the Next Question button.
Question 2 of 6
A chemical comes as a stock solution containing 20% w/v of the chemical in water. How many millilitres of the solution do you need to prepare 1 litre of 15% solution?
Type in your answer and PRESS ENTER.

No, that is not the correct answer.
Have another go: just type in your new answer and press Return.
If you continue to have problems, click on the Answer button to see the answer.
For the next question, click on the Next Question button.
Question 3 of 6
An ointment contains 60g of Benzoic Acid in 1000g of ointment. How many grams of emulsifying ointment must be added to 300g of the ointment to reduce the concentration of Benzoic Acid to 4%?
Type in your answer and PRESS ENTER.

No, that is not the correct answer.
Have another go: just type in your new answer and press Return.
If you continue to have problems, click on the Answer button to see the answer.
For the next question, click on the Next Question button.
Question 4 of 6
What is the percent w/v concentration of Morphine Sulphate in the solution when 100ml of 0.1% w/v Morphine Sulphate solution, 25ml of 0.2% w/v Morphine Sulphate solution and 50ml of a 1 in 5000 solution of Morphine Sulphate are mixed. Answer to 3 decimal places.
Type in your answer and PRESS ENTER.
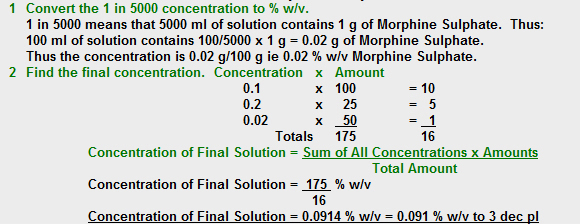
No, that is not the correct answer.
Have another go: just type in your new answer and press Return.
If you continue to have problems, click on the Answer button to see the answer.
For the next question, click on the Next Question button.
Question 5 of 6
How many grams of Coal Tar should you add to 550g of a Zinc Oxide paste in order to prepare a paste containing 12% w/w Coal Tar?
Type in your answer and PRESS ENTER.

No, that is not the correct answer.
Have another go: just type in your new answer and press Return.
If you continue to have problems, click on the Answer button to see the answer.
Question 6 of 6
You need to prepare 220 ml of a solution containing 45% Sucrose. You have 10% and 65% Sucrose solution available. How many millilitres 65% Sucrose do you need?
Type in your answer and PRESS ENTER.

No, that is not the correct answer.
Have another go: just type in your new answer and press Return.
If you continue to have problems, click on the Answer button to see the answer.