First Type of Calculation:
Finding the Concentration of a Solution or Mixture
These sort of questions can be solved by finding the weighted average of all the solutions (or mixtures).
If you mix equal amounts of a high concentration solution and a low concentration solution, the concentration of the final solution will be half way between the original two concentrations.
However, if you add more of the high concentration solution, the concentration of the final solution will be nearer that of the high concentration solution. Conversely, if you add more of the low concentration solution, the concentration of the final solution will be nearer that of the low concentration solution.
The concentration of the final solution depends on HOW MUCH of each solution is added.
If when you calculate the concentration of the final solution you bias the calculation so that the more of a solution there is present, the bigger effect it has on the average, you are WEIGHTING the average. One possible way of weighting an average is to consider the amounts (masses or volumes) added:
The concentration of the final solution depends on HOW MUCH of each solution is added.
If when you calculate the concentration of the final solution you bias the calculation so that the more of a solution there is present, the bigger effect it has on the average, you are WEIGHTING the average. One possible way of weighting an average is to consider the amounts (masses or volumes) added:
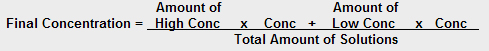
Click here to continue
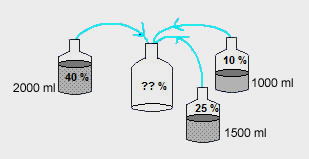
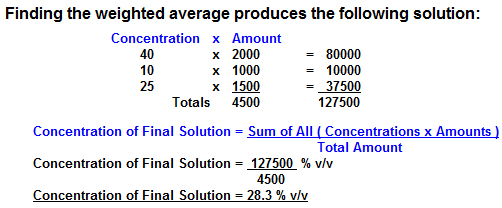
2000ml of 40% (v/v) Ethanol, 1000ml of 10% Ethanoland 1500ml of 25% Ethanol are mixed together. What is the concentration of Ethanol in the final solution ?
Click here to remove the question
Click here to continue