Section 3: Calculating the Total Number of Moles
If you know the mole fraction of a component and the number of moles of that component, you can find the total number of moles present.
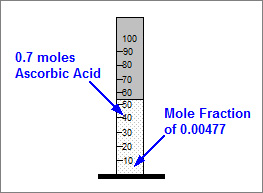
How many moles are there altogether in a solution if there are 0.7 moles of Ascorbic Acid and this is a mole fraction of 0.0047? Answer to 1 decimal place.
Click to Remove Question
There are 148.9 moles altogether.
Mole Fraction of A = Number of Moles of A
Total Number of Moles of All Components
Total Number of Moles of All Components
which rearranges to:
Total Number of Moles = Number of Moles of A
Mole Fraction of A
Total Number of Moles = 0.7 Mole Fraction of A
0.0047
Total Number of Moles = 148.93 = 148.9 moles to 1 dec pl
Click to Continue