Section 2: Calculating the Number of Moles
If you know the mole fraction of a component and the total number of moles present, you can find how many moles of the component are present.
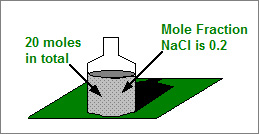
How many moles of Sodium Chloride are there in a system containing 20 moles of components if the mole fraction of Sodium Chloride is 0.2?
Click to Remove Question
There are 4 moles of Sodium Chloride present.
which rearranges to:
Moles of A = Mole Fraction of A x Total Number of Moles
Moles of Sodium Chloride = 0.2 x 20 moles
Moles of Sodium Chloride = 4 moles
Mole Fraction of A = Number of Moles of A
Total Number of Moles of All Components
Total Number of Moles of All Components
which rearranges to:
Moles of A = Mole Fraction of A x Total Number of Moles
Moles of Sodium Chloride = 0.2 x 20 moles
Moles of Sodium Chloride = 4 moles
Click to Continue