Basic Calculations in Pharmacy
| Summary |
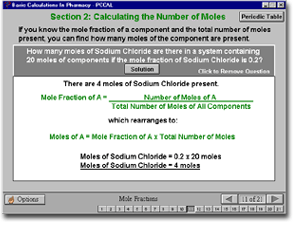
Screen shot from the package |
The package covers basic calculations and is intended
to act as a bridge between school level and first year pharmacy degree
level calculations. The types of calculation covered in the package
should already be known by the students; however experience has shown
that this is not always the case.
The aims of the package are:
- To show the students the range of calculations that they are
expected to know
- To provide help in those areas that students have commonly
found problematical.
|
| Contents |
| 1: SI Units and Terminology |
5: Conversions between Concentrations
|
- SI Units: Length
- SI Units: Mass
- SI Units: Temperature
- SI Units: Volume
- Terminology
|
|
| 2: Percentages |
6: Chemical Yields |
- Percentages
- Percent Weight in Volume
- Percent Weight in Weight
- Percent Volume in Volume
|
- Calculating Chemical Yields
|
| 3: Moles, Molarity etc. |
7: Pharmacy Practice Calculations |
- Moles and RMM
- Molarity
- Mole Fraction
- Molality
|
- Increasing/Decreasing Formulae
- Number of Doses
- Powder and Tablet Dilutions
- General Dilutions - Including Ointments
- Using Stock Solutions
- Alligation
- Least Weighable Amount / Percentage Error
|
| 4: Other Ways to Express Concentration |
- Parts Component and Ratios
- Solubility Expressions
- Normality and Equivalence
|
|
| Summary of Section |
| Subject 1: SI Units
and Terminology |
| This is a basic subject introducing
the student to the series of prefixes used for SI Units e.g. kilo-,
deci-, nano- etc., the units which are used for measuring length,
mass, temperature and volume and some of the terminology that the
students might encounter. The subject consists of five activities:
SI Units: Length, SI Units: Mass, SI Units: Temperature, SI Units:
Volume, and Terminology. |
| Subject 2: Percentages |
| This subject introduces the
student to ways of expressing concentration using percentages. Each
activity shows the student a definition, uses of the concentration
expression, how to prepare a solution of known concentration and an
equation which can be used for calculations. The activity then gives
example calculations and allows the student to practise questions.
Two methods for solving the questions are shown: the first uses the
equation itself, the second works from the definition and uses ratios
more. The subject consists of four activities: What are Percentages?
Percent Weight in Volume, Percent Weight in Weight, and Percent Volume
in Volume. |
| Subject 3: Moles,
Molarity etc. |
| This subject introduces the
student to ways to express concentration using moles. The activities
- where appropriate - show the student how to prepare a solution of
known concentration and an equation which can be used for calculations
The activities then show example calculations and allow the student
to practise questions. The subject consists of four activities: Moles
and RMM, Molarity, Mole Fraction, and Molality. |
| Subject 4: Other Ways
to Express Concentration |
| This subject looks at three
other ways to express concentration other than those covered in the
previous subjects.The subject consists of three activities: Parts
Components, Solubility Expressions, and Normality and Equivalence.
|
| Subject 5: Conversion
Between Concentrations |
| This subject contains a single
activity called Test. The activity has no explanation and consists
only of sets of questions testing whether the student can convert
between the various ways to express concentration. |
| Subject 6: Chemical
Yields |
| This subject has only one
activity called Calculating Chemical Yields. In this activity, the
student can find out how to calculate the theoretical yield of a reaction,
the percentage yield of a reaction and the minimum amount of reactant
needed to produce a specified amount of product. |
| Subject 7: Pharmacy
Practice Calculations |
| This subject covers some of
the calculations that the student should be able to do if the student
is to dispense chemicals correctly. There are six activities: Increasing/Decreasing
Formulae, Number of Doses, General Dilutions (covering dilution of
liquids and of solids), Using Stock Solutions (including Ethanol and
Formaldehyde solutions), Alligation, and Least Weighable Amount/Percentage
Error. |
| Many of the
activities within the package follow a similar structure:
First, the student is given a brief introduction to the topic:
For example in the topics on concentration, the student is given
a definition of the concentration expression, told for what the
concentration expression is used, how to prepare a solution etc.
of a particular concentration and finally some of the problems that
the student should be aware of when using that particular way of
expressing concentration.
The student is then shown an equation that can be used for calculations:
Wherever possible, the student is shown how to derive the equation
- often by using the definition given in the introduction.
For three-part equations the student then looks at how to calculate
each part of the equation:
For each part of the equation the student follows an example calculation
and can then have a go at some practice questions.
Finally, each activity finishes with a set of mixed questions:
These questions cover all the types of calculation covered in the
activity and the questions are often harder and require the student
to approach the problem in a slightly different way. |
|

Welcome to the College of Pharmacy Campus
The College is open to all pharmacy students, faculty members of pharmacy schools and to pharmacy graduates.
Click on one of the buildings to find out more about what's inside, or click the button below to log in or register.
|