For the next question, click on the Next Question button.
Question 1 of 9
grams
Convert millimoles to moles: 35 mmoles = 0.001 x 35 mmoles = 0.035 moles
Number of Moles
0.035
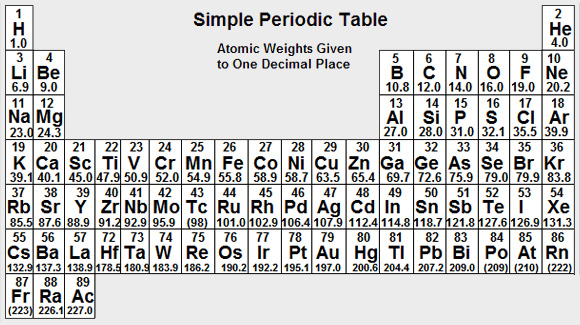
RMM of Sodium Acid Phosphate BP = 156.00
RMM of Sodium Acid Phosphate = 156.0 to one decimal place
No, that is not the correct answer.
Have another go: just type in your new answer and press Enter.
If you continue to have problems, have a look at the answer.
For the next question, click on the Next Question button.
Question 2 of 9
The following reaction takes place:
If 4.0 grams of Sodium Hydroxide were used and there was more than enough Hydrochloric Acid present, how many grams of Sodium Chloride were produced?
- Find out how many moles of NaOH were used:
Moles = Mass of Component
RMMMoles of Sodium Hydroxide = 4.0 / 40.0 = 0.1 moles -
Find out how many moles of NaCl are produced for every one mole of NaOH used.
From the equation, you can see that one mole of NaOH will result in one mole of NaCl.
- Find the number of moles of NaCl produced.
Moles of NaCl = 1 x Number of NaOH moles used
Moles of NaCl = 0.1 moles
Therefore mass of NaCl produced is Moles x RMMMass of NaCl produced = 0.1 x 58.5 grams
Mass of NaCl produced = 5.85 grams
- Find out how many moles of Sodium Hydroxide were used
- Find out how many moles of Sodium Chloride are produced for every one mole of Sodium Hydroxide used
- Find the number of moles of Sodium Hydroxide produced
You do not need to clear this hint to continue: you can just type in your answer or click on the Answer button to see the answer.
No, that is not the correct answer.
Have another go: just type in your new answer and press Enter.
If you continue to have problems, have a look at the answer.
For the next question, click on the Next Question button.
Question 3 of 9
Number of Moles = Mass of Component
RMM
Moles of Hydrochloric Acid = 21.9 / 36.5 = 0.6 moles
For every 1 mole of Magnesium Hydroxide used 2 moles of Hydrochloric Acid are used.
Hydrochloric Acid is the limiting factor and there are 0.6 moles.
3 & 4 Find out how many moles of Magnesium Chloride are produced for every mole of Hydrochloric Acid used. From the equation, you can see:
For every 2 moles of HCl used, 1 mole of Magnesium Chloride is produced. Thus:
For every 1 mole of HCl used, 0.5 moles of Magnesium Chloride are produced
Therefore Moles of Magnesium Chloride produced = 0.5 x 0.6 = 0.3 moles
Therefore Mass of Magnesium Chloride = 0.3 x 95.3 g = 28.59 grams
- Find out which of the reagents will limit the reaction
- Find out how many moles of the limiting reagent are used
- Find out how many moles of Magnesium Chloride are produced for every mole of limiting reagent used
- Find the number of moles of Magnesium Chloride produced.
You do not need to clear this hint to continue: you can just type in your answer or click on the Answer button to see the answer.
No, that is not the correct answer.
Have another go: just type in your new answer and press Enter.
If you continue to have problems, have a look at the answer.
For the next question, click on the Next Question button.
Question 4 of 9
A solution containing 23.6 grams of Succinic Acid and a solution containing 35.0 grams of Sodium Hydroxide are mixed and the following reaction occurs:
RMM Succinic Acid = 118.0 RMM Sodium Hydroxide = 40.0 RMM Sodium Succinate = 162.0
1 & 2 Find out which of the reagents will limit the reaction and how many moles of it there are.
Number of Moles = Mass of Component
RMM
Moles of Sodium Hydroxide = 35.0 / 40.0 = 0.875 moles
Succinic Acid is the limiting reagent and there are 0.2 moles.
3 & 4 Find out how many moles of Sodium Succinate are produced for every mole of Succinic Acid used.
From the equation, you can see:
For every 1 mole of Succinic Acid used, 1 mole of Sodium Succinate is produced.
Therefore Moles of Sodium Succinate produced = 0.2 moles
Therefore Mass of Sodium Succinate = 0.2 x 162.0 g = 32.4 grams
- Find out which of the reagents will limit the reaction
- Find out how many moles of the limiting reagent are used
- Find out how many moles of Magnesium Chloride are produced for every mole of limiting reagent used
- Find the number of moles of Magnesium Chloride produced.
You do not need to clear this hint to continue: you can just type in your answer or click on the Answer button to see the answer.
No, that is not the correct answer.
Have another go: just type in your new answer and press Enter.
If you continue to have problems, have a look at the answer.
For the next question, click on the Next Question button.
Question 5 of 9
If there are 39.5 grams of Potassium Permanganate (KMnO4) in 100 ml of solution, how many moles of Potassium Permanganate are there in 1000 ml of solution?
- Find out how many moles there are in 100 ml
Moles of Potassium Permanganate = 39.5 / 158.0 = 0.25 moles
- Then, using ratios find how many moles there are in 1000 ml
100 ml of solution contains 0.25 moles of Potassium Permanganate1000 ml of solution contains 1000 x 0.25 moles = 2.5 moles
100
- Using ratios, find how many grams there are in 1000 ml
100 ml of solution contains 39.5 grams of Potassium Permanganate1000 ml of solution contains 1000 x 39.5 = 395 grams of Potassium Permanganate
100 -
Then find how many moles there are in 1000 ml
Moles of Potassium Permanganate = 395 / 158.0 moles = 2.5 moles
- Find out how many moles there are in 100 ml
- Then, using ratios, find how many moles there are in 1000 ml
- Using ratios, find how many grams there are in 1000 ml
- Then find how many moles there are in 1000 ml
You do not need to clear the hint to continue. You can just type in your answer or you can click on the Answer button to see the answer.
No, that is not the correct answer.
Have another go: just type in your new answer and press Enter.
If you continue to have problems, have a look at the answer.
For the next question, click on the Next Question button.
Question 6 of 9
- Find how many grams of Ammonia there are in 1000 ml
Mass of Ammonia in 1000 ml = 0.75 x 17.0 = 12.75 grams
- Using ratios, find how many grams of Ammonia there are in 250 ml
1000 ml of solution contains 12.75 grams of Ammonia250 ml of solution contains 250 x 12.75 grams = 3.187 g = 3.19 g to 2 decimal places
1000
- Using ratios, find how many moles of Ammonia there are in 250 ml of solution
1000 ml of solution contains 0.75 moles of Ammonia250 ml of solution contains 250 / 1000 x 0.75 moles = 0.1875 moles of Ammonia
-
Find how many grams of Ammonia there are in 250 ml
Mass of Ammonia = 0.1875 x 17.0 g = 3.187 g = 3.19 g to 2 decimal places
- Find out how many grams of Ammonia there are in 1000 ml
- Using ratios, find how many grams there are in 250 ml
- Using ratios, find how many moles of Ammonia there are in 250 ml
- Find how many grams of Ammonia there are in 250 ml
You do not need to clear this hint: you can just type in your answer or click on the Answer button to see the answer.
No, that is not the correct answer.
Have another go: just type in your new answer and press Enter.
If you continue to have problems, have a look at the answer.
For the next question, click on the Next Question button.
Question 7 of 9
Number of Moles
RMM of Calcium Chloride Dihydrate = 44.13
0.3
No, that is not the correct answer.
Have another go: just type in your new answer and press Enter.
If you continue to have problems, have a look at the answer.
For the next question, click on the Next Question button.
Question 8 of 9
- Find how many grams of Sucrose are associated with 333 g water
Mass of Sucrose = 1.95 x 342.0 g = 666.9 g
- Using ratios, find how many g Sucrose associated with 1000 g water
333 g water associated with 666.9 g Sucrose1000 g water associated with 1000 / 333 x 666.9 g = 2002.70 g = 2002.7 to 1 dec pl.
- Using ratios, find how many moles of Sucrose associated with 1000 g water
333 g water associated with 1.95 moles of Sucrose1000 g water associated with 1000 / 333 x 1.95 moles = 5.8558 moles of Sucrose
-
Find how many g Sucrose associated with 1000 g water
Mass of Sucrose = 5.8558 x 342.0 g = 2002.68 g = 2002.7 g to 1 dec pl.
- Find how many grams of Sucrose are associated with 333 grams of water
- Then, using ratios, find how many grams of Sucrose are associated with 1000 grams of water.
- Using ratios, find how many moles of Sucrose are associated with 1000 g of water
- Find how many grams of Sucrose are associated with 1000 grams of water.
You do not need to clear the hint to continue: just type in your answer or click on the Answer button to have a look at the answer.
No, that is not the correct answer.
Have another go: just type in your new answer and press Enter.
If you continue to have problems, have a look at the answer.
For the next question, click on the Next Question button.
Question 9 of 9
- Find how many moles of Glucose associated with 150 g water
Moles of Glucose = 40.5 / 180.0 moles = 0.225 moles
- Using ratios, find how many moles associated with 1000 g water
150 g water associated with 0.225 moles Glucose1000 g water associated with 1000 / 150 x 0.225 moles = 1.5 moles Glucose.
- Using ratios, find how many g associated with 1000 g water
150 g water associated with 40.5 g Glucose1000 g water associated with 1000 / 150 x 40.5 g = 270.0 g Glucose
-
Find how many moles Glucose associated with 1000 g water
Moles of Glucose = 270.0 / 180.0 moles = 1.5 moles
- Find how many moles of Glucose are associated with 150 g water
- Using ratios, find how many moles Sucrose associated with 1000 g water
- Using ratios, find how many g Sucrose associated with 1000 g water
- Find how many moles of Sucrose are associated with 1000 g water.
You do not need to clear the hint to continue; you can just type in an answer or you can click on the Answer button to see the answer.
No, that is not the correct answer.
Have another go: just type in your new answer and press Enter.
If you continue to have problems, have a look at the answer.