For the next question, click on the Next Question button.
Question 1 of 3
What is the concentration of Chloride ions expressed as equivalence of a solution containing 7.1 grams of Chloride in 200 ml of solution?
Eq / litre
- Find the gram equivalent of Chloride.
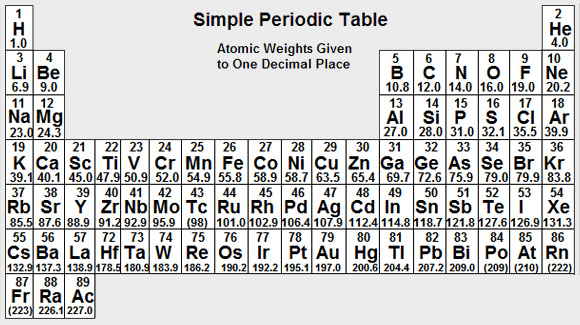
Chloride has a valency of 1 and an ionic weight of 35.5. Thus:
The gram equivalent of Chloride is 35.5 grams. -
Find the number of equivalents in 200 ml of solution.
Number of Equivalents = Total Mass of Chloride = 7.1 = 0.2 Equivalents
Chloride Gram Equivalent 35.5 -
Find the number of equivalents in 1 litre of solution.
200 ml of solution contains 0.2 Equivalents. Thus:
1000 ml of solution contains 1000/200 x 0.2 Eq = 2 Equivalents
The concentration of Chloride is 2 Eq / litre or an Equivalence of 2.
No, that is not the correct answer.
Have another go: just type in your new answer and press Enter.
If you continue to have problems, have a look at the answer.
For the next question, click on the Next Question button.
Question 2 of 3
Intracellular fluid contains 273.7 milligrams of Potassium ions in 50 ml of fluid. What is the milliequivalence of Potassium ions?
- Find the weight of one milliequivalent of Potassium.
Potassium has a valency of 1 and an ionic weight of 39.1. Thus:
The gram equivalent of Potassium is 39.1 grams. Thus:
One milliequivalent of Potassium weighs 39.1 milligrams. - Find the number of milliequivalents in 50 ml of fluid.
Number of MilliEquivalents = Total Mass of Potassium = 273.7 mEq
Weight of One Milliequivalent 39.1
-
Find the number of milliequivalents in 1 litre of solution.
50 ml of fluid contains 7 milliEquivalents. Thus:
1000 ml of fluid contains 1000/50 x 7 mEq = 140 milliEquivalents
The concentration of Potassium is 140 mEq / litre or a milliEquivalence of 140.
No, that is not the correct answer.
Have another go: just type in your new answer and press Enter.
If you continue to have problems, have a look at the answer.
For the next question, click on the Next Question button.
Question 3 of 3
250 ml of extracellular fluid contains 25 milligrams of Calcium ions. What is the milliequivalence of Calcium ions? Answer to 1 decimal place.
-
Find the weight of one milliequivalent of Calcium.
Calcium has a valency of 2 and an ionic weight of 40.1. Thus:
The gram equivalent of Calcium is 40.1/2 g = 20.05 grams. Thus:
One milliequivalent of Calcium weighs 20.05 milligrams. - Find the number of milliequivalents in 250 ml of fluid.
Number of MilliEquivalents = Total Mass of Calcium = 25 mEq
Weight of One Milliequivalent 20.05
Number of MilliEquivalents = 1.247 mEq
-
Find the number of milliequivalents in 1 litre of solution.
250 ml of fluid contains 1.247 milliEquivalents. Thus:
1000 ml of fluid contains 1000/250 x 1.247 mEq = 4.98 mEq = 5.0 mEq to 1 dec pl
The concentration of Potassium is 5.0 mEq / litre or a milliEquivalence of 5.0.
No, that is not the correct answer.
Have another go: just type in your new answer and press Enter.
If you continue to have problems, have a look at the answer.