What Happens to Oral Dosage Forms in the Body?
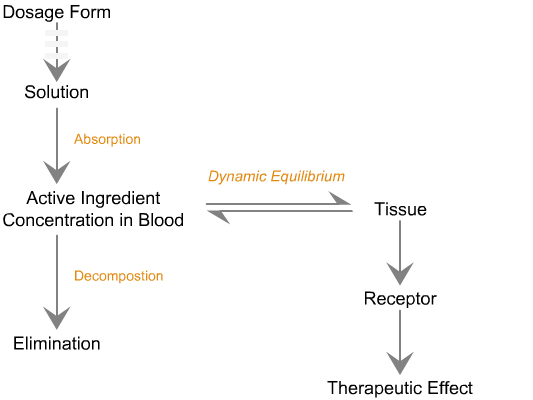
The ultimate objective of any drug is to stimulate receptors on the target tissue to generate a therapeutic effect. However, it was recognized
50 years ago that an oral dosage form must disintegrate into small aggregates and then into minute particles before the active ingredient could dissolve and be efficiently absorbed by the body.
This observation gave rise to the in vitro in vivo correlation - a predictive model describing the relationship between an in vitro property of an extended release dosage form (usually the rate or extent of drug dissolution or release) and a relevant in vivo response (such as plasma drug concentration of amount of drug absorbed).

Early pharmaceutical development strived to produce 'elegant' tablets that did not chip, deteriorate or change color. By increasing the binding agent and compression forces, tablets could be made which were less likely to fracture during packaging or shipment.
Unfortunately, these modifications reduced the formulation's disintegration abililty in the alimentary tract resulting in drug release that only occurred on the outside of the tablet. As this has a relatively low surface area when compared to its constituent particles, hardly any of the active ingredient dissolved and the drug had little effect. It is for this reason that a careful balance must be maintained whereby dosage forms readily break apart in the alimentary tract yet do not crumble during packaging or storage.
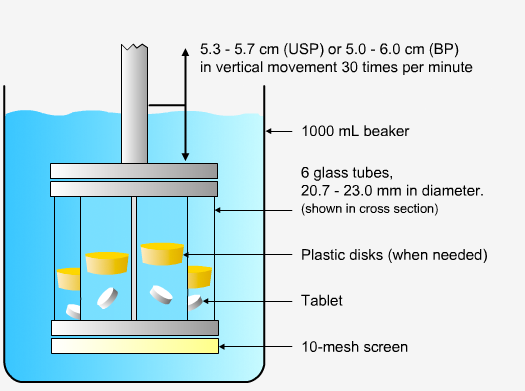
In order to measure the disintegration rate of dosage forms, specialized apparatus is required.
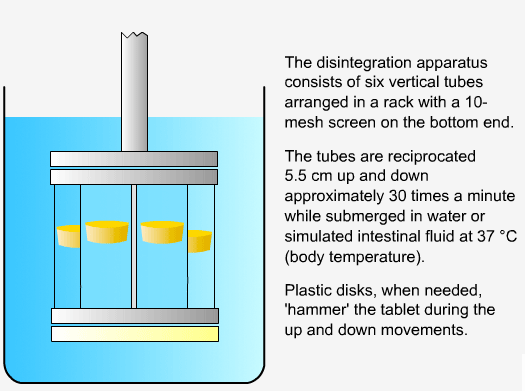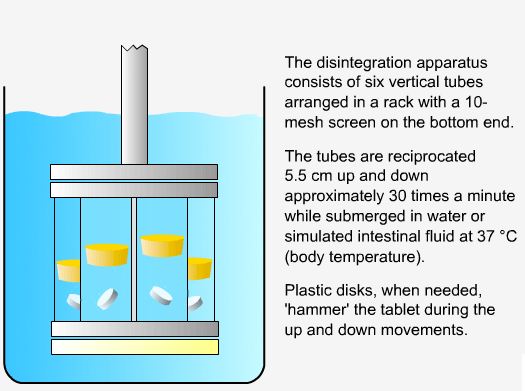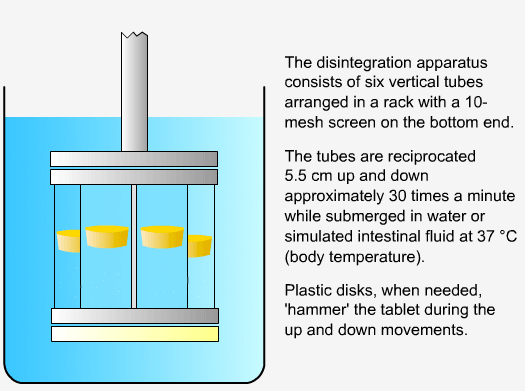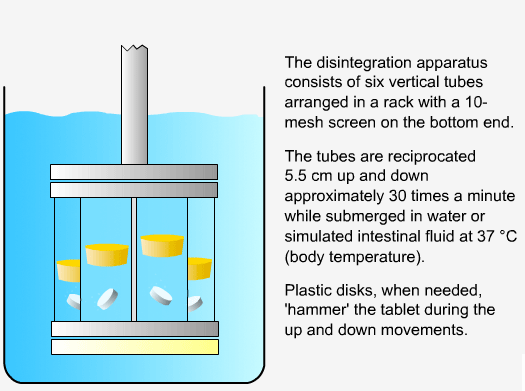
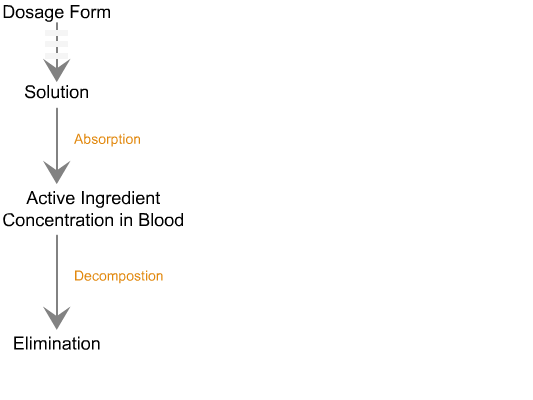
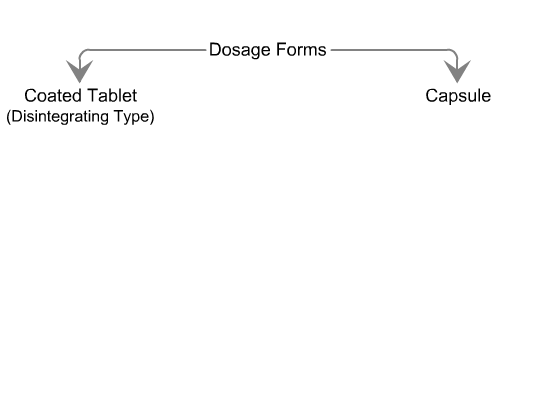
Sequence of reactions that can be readily observed in vitro
when tablets and capsules pass into solution
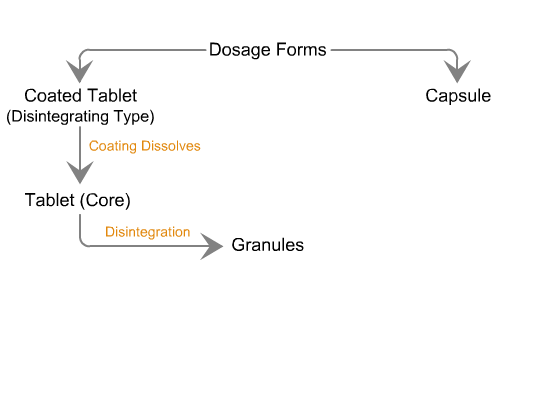
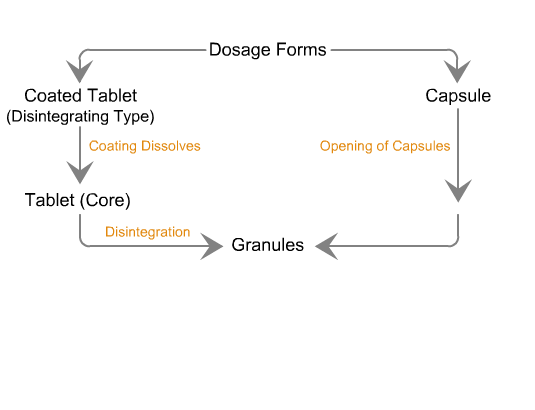
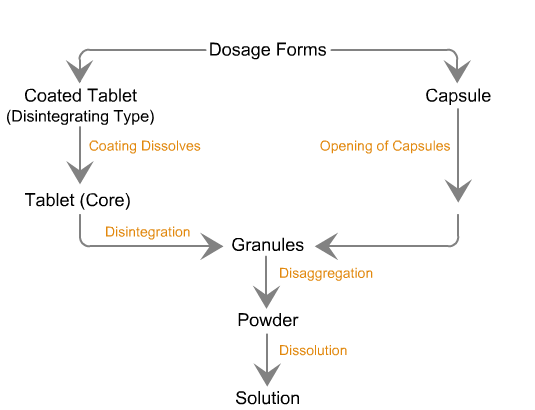
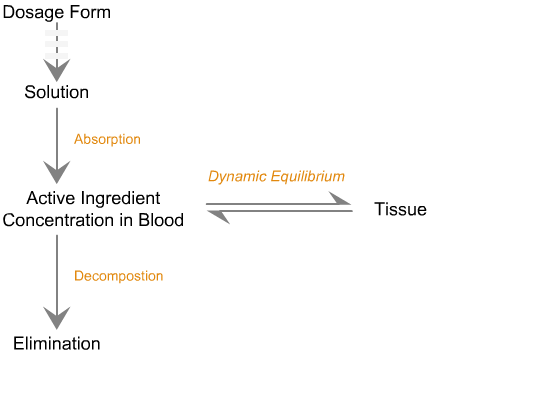
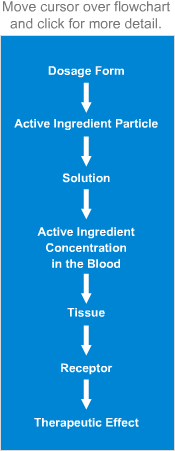
Sequence of in vivo reactions that occur
when a dosage form enters the body.
Note. The first few events are identical to what is observed in vitro. Click on the dotted arrow to expand.