Introduction To Agonists: Theory
Why Is KA An Important Constant?
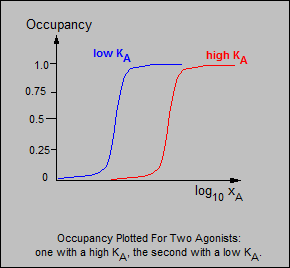
KA is an important constant because it gives an indication of
an agonist's tendency to form complexes with the receptors.
an agonist's tendency to form complexes with the receptors.
Remember, KA equals: backward rate constant
forward rate constant
forward rate constant
As KA increases, the backward rate of a reaction increases relative to the forwards rate of the reaction.
Thus a drug with a high KA has a higher backward rate of reaction than a drug with a low KA: it will therefore tend to form fewer complexes at a particular concentration.