Preparing a % w/w Product
When preparing a % w/w product, you add a specific mass of the named component and a specific mass of the other components. You do not add the other components so that the total mass is made UP TO the required mass. You can do this because masses are additive.
Look at the example below.
Compare this with a percent weight in volume preparation where you use a specific mass of the named component and then make the volume UP TO the required volume: you do not add a specific volume. Masses and volumes are not additive.

To prepare 100 grams of a cream containing 2.0 % w/w Zinc Oxide:
Find out what mass of Zinc Oxide you need.
100 grams of cream needs to contain 2 grams of Zinc Oxide.
Weigh out 2 grams of Zinc Oxide.
100 grams of cream needs to contain 2 grams of Zinc Oxide.
Weigh out 2 grams of Zinc Oxide.
Weigh out a total of 98 grams of all other components
of the cream.
of the cream.
Mix all the components together.
Check that the weight of all the components comes to 100 grams.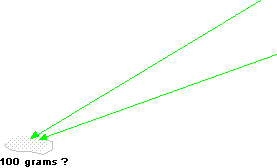
Check that the weight of all the components comes to 100 grams.

Click to Continue
Masses are Additive
If you have 10 grams of A and 20 grams of B, you know that the total mass is the sum of the two masses ie 30 grams.
You also know that if you want a total mass of 100 grams and you have 25 grams of a component, then you need to add 75 grams of other components to reach the required total.
YOU CAN ADD MASSES TOGETHER.
YOU CAN ADD MASSES TOGETHER.
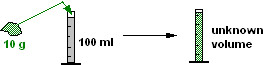
Masses and Volumes are Not Additive
If you have 10 grams of a component and you want a final volume of 100 ml, you cannot calculate what volume of the solvent to add because you do not know what volume the component will occupy once dissolved. You have to add solvent to make the volume UP TO the required amount.
MASSES AND VOLUMES CAN NOT BE ADDED TOGETHER.
MASSES AND VOLUMES CAN NOT BE ADDED TOGETHER.
Click to Continue