

You will sometimes need to work out the RMM of a compound. There is a simple Periodic Table available which you can look at by clicking on the button at the top of the screen called "Periodic Table". "To clear the table from the screen, click on "Click to Continue".
In addition, you can at any time look at the definitions of the different ways to express concentration. To do this, click on the button at the top of the screen called "Definitions". "To clear the table from the screen, click on "Click to Continue".
Click to Continue
The % w/v of any component is the number of grams of that
component contained in 100 cm3 (or 100 ml) of the total product.
The molarity of any component is the number of moles
of that component in 1000 cm3 of the total system.
of that component in 1000 cm3 of the total system.
The molality of a component in a solution is the number of moles of that component associated with one kilogram of the SOLVENT.
The mole fraction of a component within a system is the ratio
of the number of moles of that component to the total number
of moles of all components within the system.
of the number of moles of that component to the total number
of moles of all components within the system.
The Normality of a component is the number of gram equivalents
of that componet in 1000 cm3 (or 1 litre) of the total system.
The % w/w of any component is the number of grams of that component contained in 100 grams of the total product.
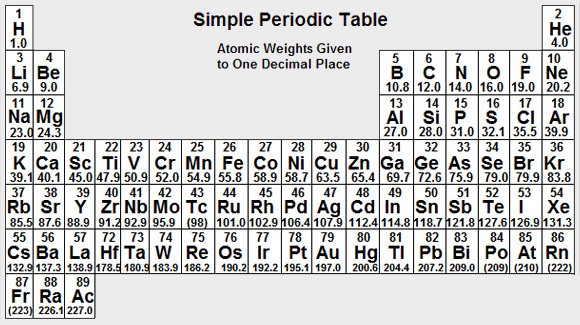
Click to Continue